Lot 22
DALTON, John (1766-1844). A New System of Chemical Philosophy. Manchester: S. Russell for R. Bickerstaff, 1808 [part 1]; Russell and Allen for R. Bickerstaff, 1810 [part 2]. FIRST EDITION, PRESENTATION COPY.
Sale 714 - Library of a Midwestern Collector
Nov 5, 2019
10:00AM CT
Live / Chicago
Own a similar item?
Estimate
$10,000 -
15,000
Price Realized
$20,000
Sold prices are inclusive of Buyer’s Premium
Lot Description
DALTON, John (1766-1844). A New System of Chemical Philosophy. Manchester: S. Russell for R. Bickerstaff, 1808 [part 1]; Russell and Allen for R. Bickerstaff, 1810 [part 2].
2 volumes, comprising parts 1 and 2 of Vol. 1 only (lacking Vol. 2 as often, published in 1827) (225 x 137 mm). 8 engraved plates. (Some light spotting to plates.) Bound in non-uniform contemporary paper-backed plain and marbled boards, printed spine labels and stencilled volume numbers, uncut (hinges starting and some light chipping to spine with a few discreet repairs to Vol. I, Pt. I, some light wear); red morocco folding case gilt. Provenance: Dr. Cleghorn (presentation inscriptions from the author); New College, Edinburgh (“Bibliotheca Coll. Nov. Edinensis” stamp on title-page).
FIRST EDITION, PRESENTATION COPY, inscribed by Dalton: “Dr. Cleghorn with respects from the Author.”
DALTON’S CLASSIC WORK ON THE ATOMIC THEORY OF MATTER IN ORIGINAL BOARDS.
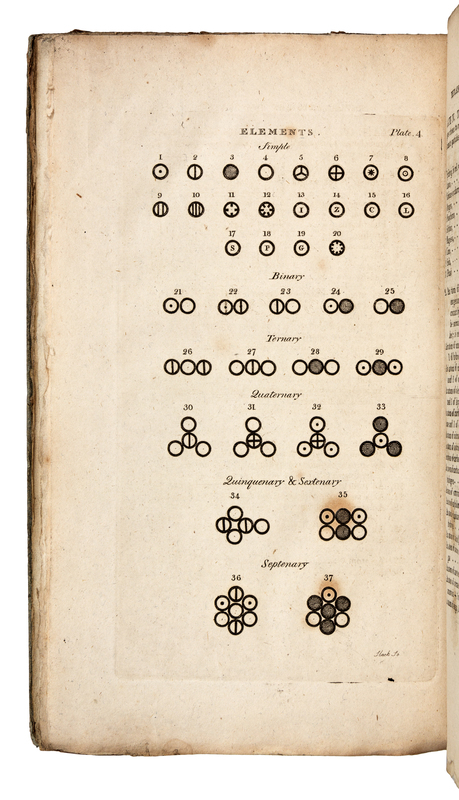
Dalton maintained that all matter was composed of indivisible atoms of various weights, and that each weight corresponded with one of the chemical elements. His was the first chemical atomic theory “to give significance to the relative weights of the ultimate particles of all known compounds, and to provide a quantitative explanation of the phenomena of chemical reaction” (Norman 575). His work prompted him to construct the FIRST PERIODIC TABLE OF ELEMENTS (plate 4 in Vol. I, part 1 facing p. 219). “Dalton reconstructed Newton’s speculations on the structure of matter, and, applying them in a new form to chemistry, gave Lavoisier’s reformation of that science a deeper significance…The identity of each atom was established by its particular weight…Hence the problem of chemical composition was that of determining how many atoms, and of what kinds, entered into the unit, later known as the molecule, of each compound substance. This problem dominated nineteenth-century chemistry” (PMM 261). “His equation of the concepts ‘atom’ and ‘chemical element’ was of fundamental importance, as it provided the chemist with a new and enormously fruitful model of reality” (Norman). Dibner Heralds of Science 44; Grolier/Horblit 22.
Condition Report
Contact Information
Auction Specialist